
Like in the displacement reactions that we looked at above, the halogens act as oxidising agents, and so their reactivity falls as you move down the group. Halogens react with hydrogen in another example of a redox reaction. 5 - Halogen displacement reactions Reactions of halogens with hydrogen Likewise, we haven't included astatine because it is both extremely rare and highly reactive, and you'll probably never come across it.įig. Halogens take part in a range of reactions. Fluorine is the most electronegative element in the periodic table. Reactivity and electronegativity decrease as you go down the group whilst atomic radius and melting and boiling point increase.

In fact, it manages to oxidise water into oxygen, complicating the reaction further. Halogen ions are called halides and are usually negative ions with a charge of -1.
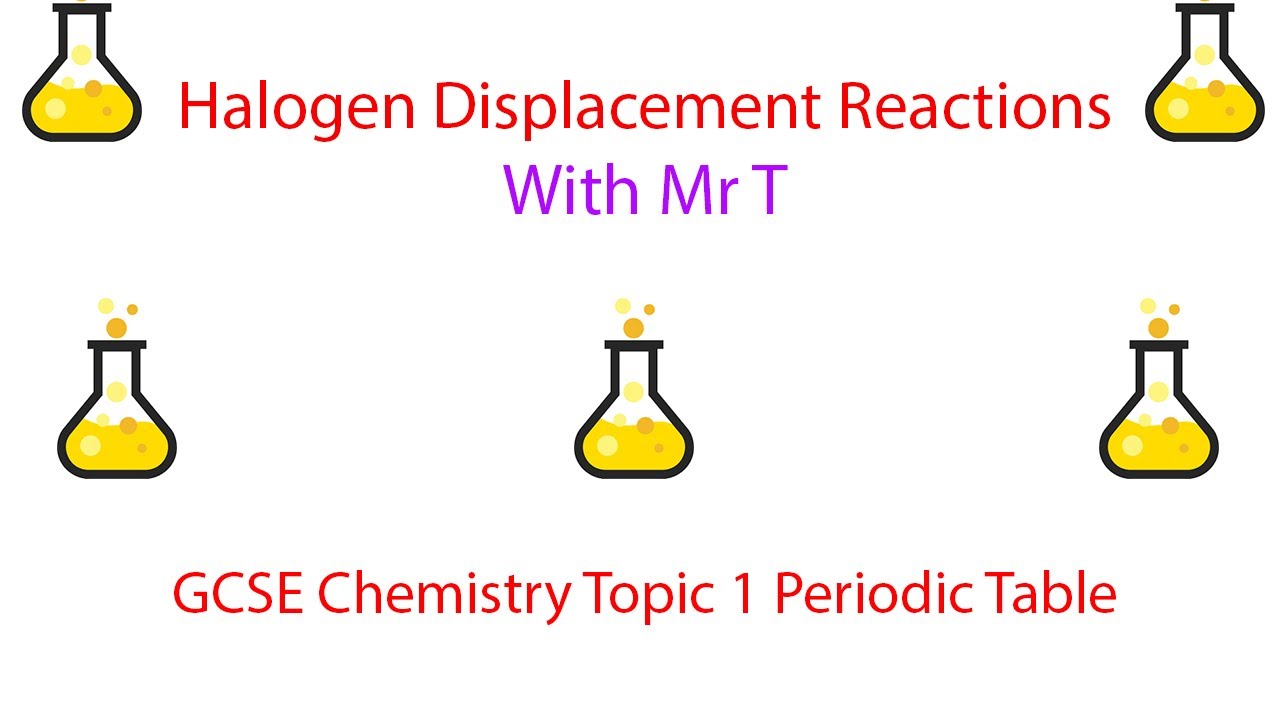
The reason is that fluorine is too strong of an oxidising agent. The halide ions lose electrons and form halogen. The following table shows the displacement reactions between different combinations of chlorine, bromine, iodine and their aqueous ions alongside any observable colour changes. Displacement reactions of halogens The more reactive halogen atoms oxidise the less reactive halide ions. It isn't a potent enough oxidising agent to oxidise the chloride ions. Bromine displaces iodide ions from solution. However, if you add bromine to a solution containing chloride ions, nothing will happen – bromine is less reactive than chlorine. As a result, the electron shielding is far greater so the nuclear force exerted on outer shell electrons is weaker, and so its easier for a halogen to take. Chlorine displaces both bromide and iodide ions from solution. The bromide ions turn the solution orange-brown. We know this reaction occurs because we can see a colour change. You can also formulate this as two half equations: Fragmentary results on other halogens (Table 4) also indicate that their reactivity integrals may be relatively low. The bromide ions form bromine, and the chlorine forms chloride ions. The bromide ions are oxidised and lose electrons, whilst the chlorine atoms are reduced and gain electrons. So chlorine atoms displace bromide ions from an aqueous solution. Bromination is selective for the R-H that gives the most.


 0 kommentar(er)
0 kommentar(er)
